Your Gut Bugs And Your Weight
Imbalances in your gut microbes (bacteria, fungi and viruses) can help to explain why you are gaining weight. Imbalances in the quantity and quality of the gut micro-organisms (microbiome) is known as dysbiosis and is now known to play a role in the cause or aggravation of many health problems, including weight excess.
Your gut is home to trillions of microorganisms which altogether are known as the gut microbiome. Every person has a unique microbiome because it is influenced by the type of birth you had, the medications you have ever taken, your diet, your environment, and your lifestyle.
Your gut bacteria influence your hunger, metabolism, inflammation, immune function, digestion, and mental wellbeing. You can do things to positively influence this gut ecosystem.
The types of food you eat greatly influence your microbiome and this can affect the tendency to gain weight or stay lean and healthy. A healthy balanced microbiome can help you to maintain a normal body weight.
A large variety of gut bugs (gut microbiome diversity) is beneficial because different types of bacteria do different things, which can affect metabolism and reduce weight gain.
You may have fewer types of bacteria (low diversity). You can influence this with what you eat, and it can help explain weight gain. Many scientific studies show that lack of diversity in the microbiome is common amongst overweight and obese individuals. Some microbes help protect us from weight gain and obesity by strengthening the gut lining and maintaining healthy metabolic markers.
Research on the microbiome of overweight people
Bacteria break down food particles into energy they require for themselves to exist and they leave behind metabolites.
The good bugs (beneficial gut microbes) break down the complex carbohydrates in whole plant foods by fermentation and this produces short-chain fatty acids (SCFAs), vitamins, and other metabolites.
The SCFAs are an important energy source for the gut, and they are also signalling molecules. SCFAs send messages to the major gut nerve (vagus nerve) that sends messages from your gut to your brain, and this can affect both food intake and metabolism.
Acetate and butyrate are two SCFAs that bind to receptors on the cells lining your gut and help regulate fat storage and control appetite. These receptors also cause the release of the gut hormones that regulate appetite: peptide YY and GLP-1. In normal amounts, they reduce appetite, but if deficient you could feel the need to snack more often and too much.
Slim bacteria
Akkermansia muciniphila eats the mucus that covers your gut lining, which stimulates the lining to produce more mucus. This makes it stronger and thicker, reducing unwanted metabolites and toxins from entering the blood stream where they can cause immune system problems. Akkermansia muciniphila is more abundant in lean people and less abundant in overweight people. Research shows that Akkermansia muciniphila can help the body control fat and sugar metabolism, which are important for the process of weight loss. Another good bacterium called Christensenella minuta has been detected in greater amounts in lean people. In mice studies, Christensenella minuta has been shown to reduce weight gain.
Obesity and a diet of high-sugar foods are linked with a less diverse microbiome
A healthy diverse balanced gut microbiome is linked to a healthy weight which is not surprising as it maintains the gut lining and regulates appetite hormones.
There are specific patterns of imbalances in gut bacteria (dysbiosis) in obese individuals. People who are overweight tend to have a reduced abundance of Bacteroidetes and an increase in Firmicutes.
The Bacteroidetes-to-Firmicutes ratio is linked with a greater extraction of energy from food, which can lead to elevated blood sugar and fat levels
Unprocessed high fibre foods like vegetables, nuts and seeds are digested slowly and feed your gut bacteria because many of their components can’t be broken down by human digestive enzymes and only by the gut bacteria.
A diet high in processed foods and sugar can lower microbiome diversity and encourage the less beneficial bacteria to thrive.
Thus, diet is a major factor in the diversity and health of your microbiome. Having lots of different gut bacteria keeps the gut ecosystem balanced.

Foods to nourish the beneficial bacteria in your gut
- Vegetables
- Fruit in moderation and low carbohydrate varieties are best
- Garlic
- Cranberry
- Jerusalem artichoke
- Blackberry
- Mushrooms
- Citrus
Try to get at least 30g of fibre every day from whole foods. Research also shows that people who eat more than 30 different plants of the food rainbow (red, orange, yellow, green, and purple/blue) every week have the greatest microbiome diversity.
Dysbiosis
Dysbiosis is characterized by:
- Reduced diversity (variety) of bacteria and excess amounts of unhealthy micro-organisms (bacteria, viruses and fungi) in the gut
- Excess inflammation
Many factors affect the gut microbiome such as diet, gut infections, physical activity, medications, host genetics, and the geographic living area.
Leaky gut can affect the body in many ways and is caused by an injured intestinal barrier (the mucus membrane which lines the inside of the gut).
An injured intestinal barrier allows the penetration of bacteria and/or bacterial products through the barrier into the blood stream and is an important cause of fatty liver and weight gain. Obesity is associated with chronic inflammation, and some antigens (proteins) from the gut may be inducers of inflammation.
The gut microbiome influences distant organs, including the liver, pancreas and the adipose (fat) tissue. Toxic metabolic products produced by unhealthy gut bacteria can increase insulin resistance in the fat tissue causing weight gain.
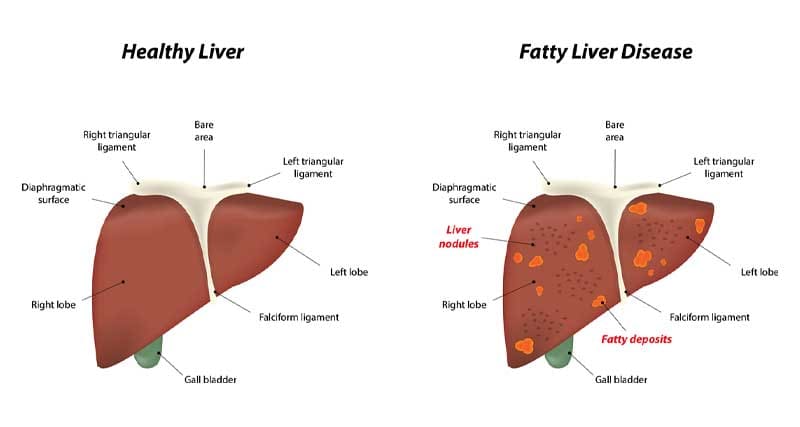
The liver is exposed to signals from the digestive tract and gut microbiome
Imbalances in the gut microbiome are connected with a higher risk for liver disorders associated with obesity, such as non-alcoholic fatty liver disease (NAFLD) [4]. The severity of NAFLD and cirrhosis of the liver is associated with the degree of unhealthy (dysbiotic) gut bacteria [19].
A dysbiotic gut microbiome resulted in the accumulation of triglyceride fats in the liver [3]. The gut microbiome has a large influence on the metabolism of bile acids and if disturbed may induce metabolic dysfunction, obesity and insulin resistance.
Ethanol and other bacterial products associated with the worsening of NAFLD may be related to a high amount of alcohol producing bacteria from the Proteobacteria genus of gut bacteria.
How cooking certain foods might alter the gut microbiota
A study published in Nature Microbiology explored how eating cooked food versus raw food affects the gut microbiome of humans.
Studies in mice found that those fed raw vegetables lost more weight than cooked-fed mice. This was thought to be because consumption of cooked vegetables impacted energy intake due to the greater digestibility of starch from the cooking process. This prompted the researchers to test the gut microbiota of five healthy women and three healthy men aged 24–40 who ate either raw or cooked plant-based meals for three consecutive days.
The researchers discovered that like mice, the study participants showed subtle but distinct differences after eating raw or cooked foods. The shifts observed in the gut microbiome could be due to the fact that cooking changes the physical structure of naturally occurring compounds found in foods making their calories more absorbable. Ref 26
Although the number of participants in this study was small, it shows the significance of controlling cooking method and not just nutrient intake when investigating the diet-gut microbiome connection.
Research into the links between cooking methods and gut health and weight gain is still in its infancy and more large-scale studies are needed to better understand how cooking foods could potentially affect weight.
There is a lot of controversy surrounding the safety of microwave ovens. Microwaving food is very different to traditional cooking, which transfers heat from within one object to the outside of another, which then penetrates within. Microwaves use radio-like waves (radiation) to generate heat from water inside the food. Excited particles within the food cells then cook your food. Using radiation to cook food raises concern in me and several of my scientific colleagues. After microwaving food it’s still cooking and you are ingesting active radio waves that may come into contact with intestinal bacteria and other microbes (the intestinal flora). Conventional science refutes that theory but it is controversial. Some research does show that microwaving foods may retain more nutrients as compared to more extended cooking methods, however other research shows that microwaves lessen the nutritional value of vegetables.
Microwave cooking is often associated with processed TV Dinners and supermarket off the shelf frozen meals high in preservatives; these are not conducive to a healthy gut microbiome.
It is proven that microwaving breast milk causes severe damage to its properties so this can be logically extended to other foods. Solid scientific evidence shows that breastmilk begins to lose its “superfood powers” when heated past 104 degrees F. Here are the ways that human milk deteriorates as it gets too hot in a microwave:
- Immunologic and anti-inflammatory components (such as IgA antibodies, lactoferrin protein, and lysozyme (infection protectant)) are decreased.
- Beneficial probiotic bacteria are destroyed.
- White blood cells are destroyed, in turn decreasing anti-infective properties.
- Fat content decreases which is vital for infant growth
- Inactivation of digestive enzymes.
If this occurs in breast milk it can happen to other foods which can become similarly devitalized. Damaged food is not conducive to a robust diverse microbiome.
How medications can affect the gut microbiome
I once saw an overweight man with a fatty liver and indigestion who had been on antacid drugs for over a decade without a break. The drugs are called Proton Pump Inhibitors (PPIs) and are taken by millions of people. He complained of fatigue, abdominal obesity and reflux. He showed me a photograph of the pictures of his stomach and small intestine that were taken during his recent gastroscopy. I was shocked! His stomach and duodenum were covered by a thick white layer of the yeast known as candida. His gut microbiome was overtaken by infection with candida and this was causing him severe problems. We treated the yeast infection and put him on a low carbohydrate diet and the natural antibiotic called BACTOCLEAR and he no longer needed the antacid drugs. He also found that his liver function improved and he lost the excess abdominal weight.
A lack of stomach acid production is a common cause of dysbiosis in older people or those with gastritis (stomach inflammation). It is vital to have adequate production of hydrochloric acid in the stomach as it acts as a natural antibiotic to prevent the bad bacteria from taking over. Many people with low production of stomach hydrochloric acid can benefit from taking supplements of hydrochloric acid (betaine HCL) in the middle of their meal and will find their gut microbiome improves a lot. This can make it easier to lose weight.
In conclusion
Research has shown that long-term weight gain in humans is related to low microbial diversity within the gut. This lack of diversity is encouraged by a low fibre diet high in processed foods. Try to eat unprocessed foods and cook them by using conventional methods such as roasting, baking, stir fry or steaming. Wash your raw produce well to remove unhealthy bacteria. Having a healthy gut microbiome will help to protect you from dangerous weight excess.
Dr Sandra Cabot and naturopath Victoria Taylor have done a podcast on “The connection between the gut and the weight”.
Scientific references:
De Clercq NC, et al, Gut Microbiota in Obesity and Undernutrition. The Advances in Nutrition journal. 2016;15(7(6)):1080–1089.https://doi.org/10.3945/an.116.012914 PMid:28140325 PMCid:PMC5105041. [PMC free article] [PubMed] [Google Scholar]
2. Parekh PJ, et al, The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. The Frontiers in endocrinology journal. 2014;5:47. https://doi.org/10.3389/fendo.2014.00047 PMid: 24778627 PMCid:PMC3984999.
3. Bäckhed F, et al. Mechanisms underlying the resistance to diet induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):979–84.https://doi.org/10.1073/pnas.0605374104 PMid:17210919 PMCid:PMC1764762.
4. Turnbaugh PJ, et al, An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.https://doi.org/10.1038/natur.e05414 PMid:17183312.
5. Ridaura VK, et al, Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. https://doi.org/10.1126/science.1241214 PMid:24009397 PMCid:PMC3829625.
6. Aagard K, et al, The Human Microbiome project strategy for comprehensive sampling of the human microbiome and why it matters. The FASEB Journal. 2013;27(3):1012–22. https://doi.org/10.1096/fj.12-220806 PMid:23165986 PMCid:PMC3574278
7. Huttenhower C et collaborators. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14.https://doi.org/10.1038/natur.e11234 PMid:22699609 PMCid:PMC3564958.
8. Methe BA et collaborators. A framework for human microbiome research. Nature. 2012;486(7402):215–21.https://doi.org/10.1038/natur.e11209 PMid:22699610 PMCid:PMC3377744.
9. Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. The Gastroenterology journal. 2014;146(6):1449–58.https://doi.org/10.1053/j.gastro.2014.01.052 PMid:24486050 PMCid:PMC4181834.
10. Andersson AF, et al, Comparative analysis of human gut microbiota by barcoded pyrosequencing. The PloS One Journal. 2008;3(7): e2836.https://doi.org/10.1371/journal.pone.0002836 PMid:18665274 PMCid:PMC2475661
11. Jameson JL, et al, The Human Microbiome. 20th edition. McGraw-Hill Education; 2018. Harrison’s principles of Internal medicine; pp. 3379–3390
12. Passos MDCF, Moraes-Filho JP. Intestinal microbiota in digestive diseases. The Arquivos de gastroenterologia. 2017;54(3):255–262. https://doi.org/10.1590/s0004-2803.201700000-31 PMid:28723981.
13. Fauci AS, Braunwald E, Kasper DL, Stephen LH, Longo DL, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine 17th edition:Biology of obesity. McGrawHill Medical. 2008:462–468. [Google Scholar]
14. Turnbaugh PJ, et al, A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4.https://doi.org/10.1038/nature07540 PMid:19043404 PMCid:PMC2677729. [PMC free article] [PubMed] [Google Scholar]
15. Ley RE, et al, Microbial ecology:human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. https://doi.org/10.1038/4441022a PMid:17183309.
16. Sun L, Ma L, et al, Insights into the role of gut microbiota in obesity:pathogenesis, mechanisms and therapeutic perspectives. The Proetin & Cell journal. 2018;9(5):397–403. https://doi.org/10.1007/s13238-018-0546-3 PMid:29725936 PMCid:PMC5960470.
17. Kimura I, et al, The gut microbiota suppresses insulin-mediated fat accumulation via short-chain fatty acid receptor GPR43. The Nature communications journal. 2013;4:1829.https://doi.org/10.1038/ncomms2852 PMid:23652017 PMCid:PMC3674247.
18. Nohr MK, et al, GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. The Endocrinology journal. 2013;154(10):3552–64. https://doi.org/10.1210/en.2013-1142 PMid:23885020.
19. Boursier J, et al, The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. The Hepatology journal. 2016;63(3):764–75.https://doi.org/10.1002/hep.28356 PMid:26600078 PMCid:PMC4975935.
20. Breton J, et al, Gut Commensal E.coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. The Cell Metabolism journal. 2016;23(2):324–34. https://doi.org/10.1016/j.cmet.2015.10.017 PMid:26621107
21. Queipo-Ortun˜o MI, et al, Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. The PloS one journal. 2013;8(5):e65465.https://doi.org/10.1371/journal.pone.0065465 PMid:23724144 PMCid:PMC3665787
22. Blaser MJ. Hypothesis:the changing relationship of Helicobacter pylori and humans: implications for health and disease. The journal of infectious diseases. 1999;179(6):1523–30.https://doi.org/10.1086/314785 PMid:10228075.
23. Cho I, et al, Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6.https://doi.org/10.1038/natur.e11400 PMid:22914093 PMCid:PMC3553221.
24. Neish AS. Microbes in gastrointestinal health and disease. The Gastroenterology journal. 2009;136(1):65–80. https://doi.org/10.1053/j.gastro.2008.10.080 PMid:19026645 PMCid: PMC2892787.
25. Vulevic J, et al, A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. The Journal of nutrition. 2013;143(3):324–31.https://doi.org/10.3945/jn.112.166132 PMid:23303873.
26. Carmody RN, et al. Cooking shapes the structure and function of the gut microbiome. Nature Microbiology, 2019. doi: 10.1038/s41564-019-0569-4 https://www.nature.c om/articles/s41564-019-0569-4

Leave A Comment